Medtronic Resolute Onyx Coronary Drug Eluting Stent
$ 0.00 – $ 2127.09Price range: $ 0.00 through $ 2127.09
Features:
Medtronic Resolute Onyx Coronary Drug Eluting Stent
Continuous Sinusoid Technology
Novel MicroTrac Delivery System
Core Wire Technology
Enhanced Visibility
| Title | Quantity | Price |
|---|---|---|
| Bulk/tiered discount | 2 - 5 | 1% $ 0.00 |
| Bulk/tiered discount | 6 - 10 | 2% $ 0.00 |
| Bulk/tiered discount | 11 - 20 | 4% $ 0.00 |
| Bulk/tiered discount | 21 - 50 | 5% $ 0.00 |
| Bulk/tiered discount | 51 + | 7% $ 0.00 |
The Medtronic Resolute Onyx Coronary Drug-Eluting Stent is an advanced workhorse DES ready for your challenging coronary cases
It combines Continuous Sinusoid Technology and Core Wire Technology in a novel manufacturing process that offers thinner struts, exceptional deliverability, and enhanced visibility without compromising structural strength.
The Resolute Onyx DES further evolves the flexible stent platform achieved by Continuous Sinusoid Technology
It features a predictable performance with sustained structural and coating integrity, and minimal foreshortening, even at maximum overexpansion.
It has a platinum-iridium core within a cobalt alloy shell which enhances radiopacity for more accurate stent placement
Indications:
The Resolute Integrity Zotarolimusluting Coronary Stent System is indicated for improving coronary luminal diameters in patients, including those with diabetes mellitus, with symptomatic ischemic heart disease due to de novo lesions of length 5 mm in native coronary arteries with reference vessel diameters of 2.25 mm to 4.20 mm
Precautions:
Only physicians who have received adequate training should perform implantation of the stent
Stent placement should only be performed at hospitals where emergency coronary artery bypass graft surgery can be readily performed
Subsequent stent restenosis or occlusion may require repeat catheter-based treatments (including balloon dilatation) of the arterial segment containing the stent. The long-term outcome following repeat catheter-based treatments of previously implanted stents is not well characterized.
The risks and BENEFITS of the stent implantation should be assessed for patients with a history of severe reaction to contrast agents
Do not expose or wipe the product with organic solvents such as alcohol
When drug-eluting stents (DES) are used outside the specified Indications for Use, patient outcomes may differ from the results observed in the RESOLUTE pivotal clinical trials.
Compared to use within the specified Indications for Use, the use of DES in patients and lesions outside of the labeled indications, including more tortuous anatomy, may have an increased risk of adverse events, including stent thrombosis, stent embolization, myocardial infarction (MI) or death.
Care should be taken to control the position of the guide catheter tip during stent delivery, deployment and balloon withdrawal. Before withdrawing the stent delivery system, visually confirm complete balloon deflation by fluoroscopy to avoid guiding catheter movement into the vessel and subsequent arterial damage.
Stent thrombosis is a low-frequency event that is frequently associated with MI or death. Data from the RESOLUTE clinical trials have been prospectively evaluated and adjudicated using the definition developed by the Academic Research Consortium (ARC)
The stents are available in 9 lengths and 9 diameters
1. We offer Worldwide Shipping by Courier via Air or Sea mode.
2. Product will be dispatched within 5-7 working days.
3. Delivery will take a maximum of 7-15 days, based on the shipping option you choose.
4. Kindly check customs restrictions and rules in your country for specific products, mostly 99% shipments are cleared and delivered smoothly.
5. For Bulk inquiries or customized manufacturing please contact us.
Product Enquiry
- Veterinary
- Agro Chemicals
- Category
- Medical Supplies
- Dialysis Supplies
- Mobility Aids
- Anatomical Models
- Point of Care Testing
- Cath Lab Products
- Gloves
- Medical Simulators
- Rehabilitation
- Medical Accessories
- Cleaning and Waste Management
- Hearing Aids
- Medical Clothing
- Respiratory & Anaesthesia Supplies
- Hospital Apparel, Linen and Personal Protection
- Sterilization, Antiseptics & Disinfectants
- IV, Infusion & Transfusion
- Surgical Instruments
- Medical Equipments
- Surgical Supplies
- Braces, Splints & Supports
- Medical Instruments
- Surgical Sutures
- Body Weight Machine
- Needles & Syringes
- Dental Supplies
- Urology, Ostomy & Incontinence
- Gynecology & Infant Care
- Respiratory Care Products
- Nursing Supplies/Patient Care
- Health & Nutrition
- Wound Care & Dressings
- Medical Equipment
- Ophthalmic Supplies
- Diagnostic Instruments
- Diagnostic & Imaging Supplies
- Orthopaedic & Trauma
- Cardio Thoracic Surgery
- Dental Products
- Clinical Problem Solvers
- Dental Consumables
- Orthodontics
- Oral Surgery
- Student Section
- Periodontics
- Cosmetic Dentistry
- General Dentistry
- Sterilization
- Endodontics
- Kits
- Medical Supplies
- Equipments
- Laboratory
- Implantology
- Instruments
- Basic Dental Products
- Paedodontics
- Preventives
- Corona Safety
- Prosthodontics
- Rative Next
- Dental Furniture
- Dental Equipment
- Restoratives
- Patient Education
- Offers
- Dental Models
- Lab Supplies
Related Products
Features:
3M Avagard Hand Sanitizer
Size: 100/500ml/2.5L
With/Without Pump
Prevent dryness
Optimum Moisturising
Out of stock
Features:
3M Aura Healthcare Particulate Respirator and Surgical Mask
For Healthcare Facilities
Colour: White
FDA Cleared
Features:
3M Cavit G Temporary Filling Material
Volume: 28g
Quick & Void-free Curing
Completely Removable without Burs
Simple to Apply
Features:
3M Cavilon No Sting Barrier Film Wand
Volume: 1/3 ml
Type: Wand
Box of 25
Alcohol free
Features:
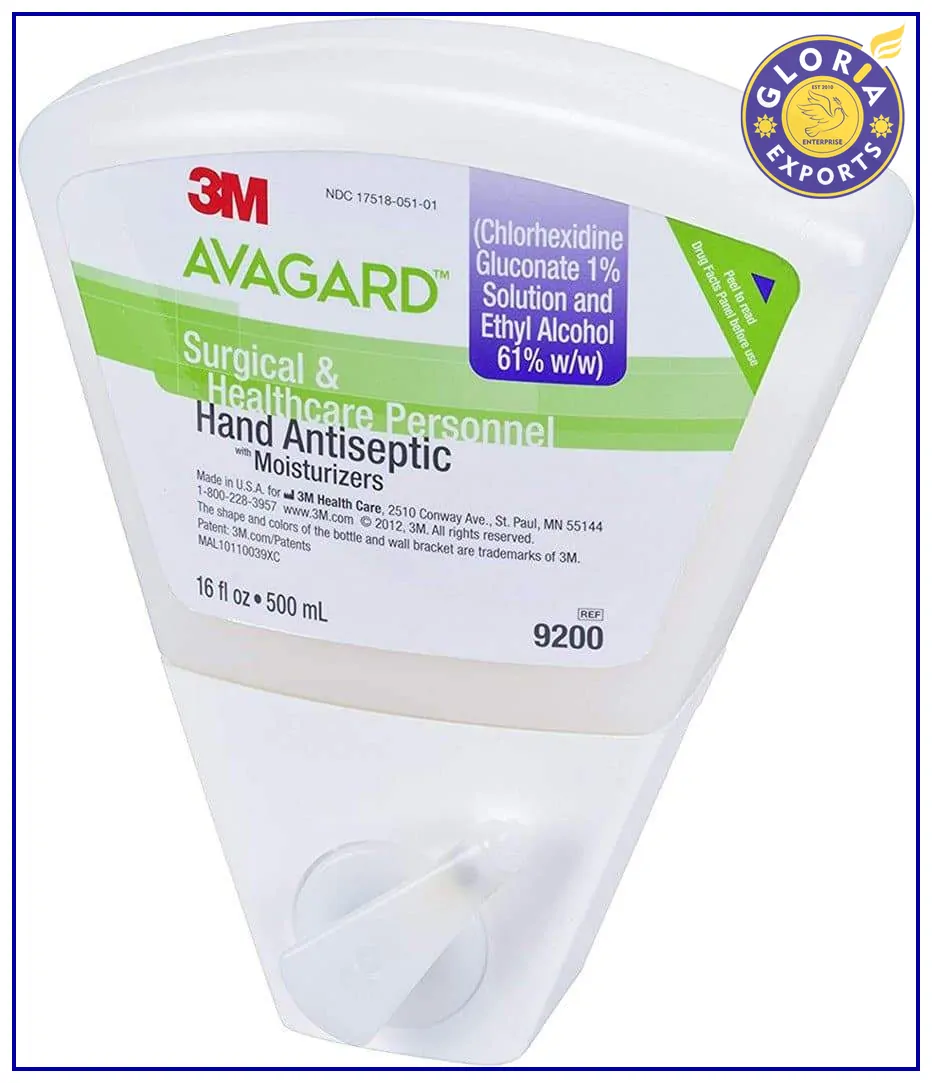
3M Avagard Surgical Hand Antiseptic Hand Sanitizer
Size: 500/1200 ml
Dispenser/Wedge bottle
Effectively kills bacteria
Skin friendly
Out of stock
Features:
3M Durapore Silk Surgical Tape
Available in 4 sizes
Silk-like Material
Conformable
Hypoallergenic & latex free
Features:
3M Avagard Surgical Scrub CHG 4%
Size: 500ml/100ml
Surfactants & moisturisers
Broad spectrum
Added moisturizers
Features:
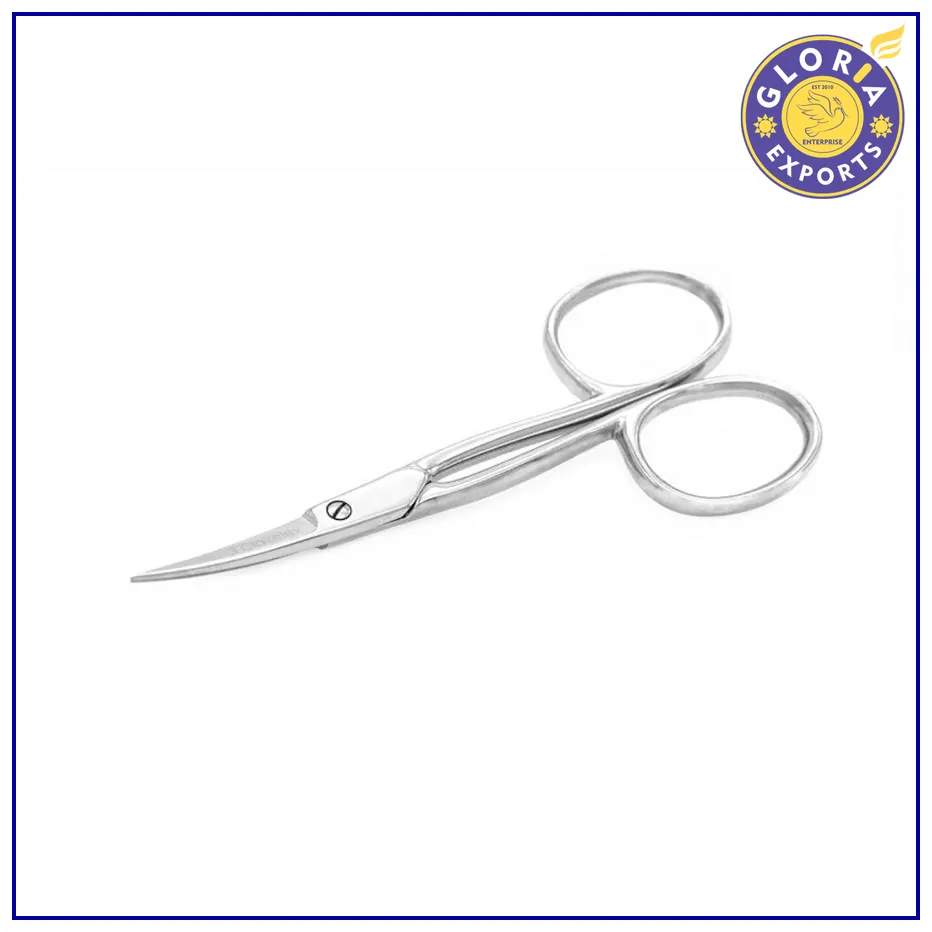
3M Curved Crown Scissors
Type: Curved
Material: Stainless steel
Lightweight
Easy to Use
Features:
3M Comply Autoclave Steam Indicator Tape
Size: 18mmX55m
Lead free
Steam Indicator Tape
Easy to apply
Features:
3M Aplicap Activator/Applier Set
Set Includes 2 Items
For Easy Activation
Lightweight
Durable
Features:
3M Clinpro Dental Sealant
Long-lasting Protection Against Caries
Colour Change Technology
Low Viscosity
Bonds to Enamel
Features:
3M Avagard CHG 500 ml Hand Sanitizer – Pack of 10
Size: 500 ml
Rapid skin disinfection
Kills 99.99% of microbes
Protection for longer duration
Out of stock