Ethicon Endo Flexipath Thoracic Trocars
$ 101.16 – $ 101.30Price range: $ 101.16 through $ 101.30
Features:
Ethicon Endo Flexipath Thoracic Trocars
Single Patient Use
Flexible Sleeve
Mechanical Obturator
With Stability Threads
| Title | Quantity | Price |
|---|---|---|
| Bulk/tiered discount | 2 - 5 | 1% $ 100.15 |
| Bulk/tiered discount | 6 - 10 | 2% $ 99.14 |
| Bulk/tiered discount | 11 - 20 | 4% $ 97.11 |
| Bulk/tiered discount | 21 - 50 | 5% $ 96.10 |
| Bulk/tiered discount | 51 + | 7% $ 94.08 |
Ethicon Endo Flexipath Thoracic Trocars are sterile singleatientse instruments that consist of a mechanical obturator and a flexible sleeve
The flexible sleeve incorporates holes (for sutures or skin staples) to secure it to the skin
The Flexipath Flexible Surgical Trocar has application in thoracoscopic and other minimally invasive procedures to establish a port for access to internal organs where insufflation is not required
The instrument is not intended for use when minimally invasive techniques are contraindicated or when insufflation is necessary
These sterile, single patient use instruments are available in 3 diameters
These trocars are available in the length of 80 mm
Warnings & Precautions:
Minimally invasive procedures should be performed only by persons having adequate training and familiarity with minimally invasive techniques. Consult medical literature relative to techniques, complications and hazards prior to performance of any minimally invasive procedure.
Minimally invasive instruments may vary in diameter from manufacturer to manufacturer. When minimally invasive instruments and accessories from different manufacturers are employed together in a procedure, verify compatibility prior to initiation of the procedure.
A thorough understanding of the principles and techniques involved in laser, electrosurgical, and ultrasonic procedures is essential to avoid shock and burn hazards to both patient and medical personnel and damage to the device or other medical instruments. Ensure that electrical insulation or grounding is not compromised. Do not immerse electrosurgical instruments in liquid unless the instruments are designed and labeled to be immersed
After removing the instrument from the thoracic cavity, inspect the site for hemostasis. If hemostasis is not present, appropriate techniques should be used to achieve hemostasis
Once partial entry has been accomplished, very little pressure may be required to complete entry. Excessive pressure could cause injury to intrabdominal or intrahoracic structures.
Instruments or devices which come into contact with bodily fluids may require special disposal handling to prevent biological contamination.
Dispose off all opened instruments whether used or unused.
This device is packaged and sterilized for single use only. Do not reuse, reprocess or resterilize. Reuse, reprocessing, or resterilization may compromise the structural integrity of the device and/or lead to device failure that in turn may result in patient injury, illness or death. Also, reprocessing or resterilization of single use devices may create a risk of contamination and/or cause patient infection or crossnfection, including, but not limited to, the transmission of infectious disease(s) from one patient to another. Contamination of the device may lead to injury, illness, or death of the patient
1. We offer Worldwide Shipping by Courier via Air or Sea mode.
2. Product will be dispatched within 5-7 working days.
3. Delivery will take a maximum of 7-15 days, based on the shipping option you choose.
4. Kindly check customs restrictions and rules in your country for specific products, mostly 99% shipments are cleared and delivered smoothly.
5. For Bulk inquiries or customized manufacturing please contact us.
Product Enquiry
- Agro Chemicals
- Category
- Medical Supplies
- Cath Lab Products
- Gloves
- Medical Simulators
- Rehabilitation
- Medical Accessories
- Cleaning and Waste Management
- Hearing Aids
- Medical Clothing
- Respiratory & Anaesthesia Supplies
- Hospital Apparel, Linen and Personal Protection
- Sterilization, Antiseptics & Disinfectants
- IV, Infusion & Transfusion
- Surgical Instruments
- Medical Equipments
- Surgical Supplies
- Braces, Splints & Supports
- Medical Instruments
- Surgical Sutures
- Body Weight Machine
- Needles & Syringes
- Dental Supplies
- Urology, Ostomy & Incontinence
- Gynecology & Infant Care
- Respiratory Care Products
- Nursing Supplies/Patient Care
- Health & Nutrition
- Wound Care & Dressings
- Medical Equipment
- Ophthalmic Supplies
- Diagnostic Instruments
- Diagnostic & Imaging Supplies
- Orthopaedic & Trauma
- Cardio Thoracic Surgery
- Dental Products
- Orthodontics
- Oral Surgery
- Student Section
- Periodontics
- Cosmetic Dentistry
- General Dentistry
- Sterilization
- Endodontics
- Kits
- Medical Supplies
- Equipments
- Laboratory
- Implantology
- Instruments
- Basic Dental Products
- Paedodontics
- Preventives
- Corona Safety
- Prosthodontics
- Rative Next
- Dental Furniture
- Dental Equipment
- Restoratives
- Patient Education
- Offers
- Dental Models
- Clinical Problem Solvers
- Dental Consumables
- Lab Supplies
- Dialysis Supplies
- Mobility Aids
- Anatomical Models
- Point of Care Testing
- Veterinary
Related Products
Features:
3M Attest Biological Indicator 1264 for EO Sterilization
Box of 100
Biological indicator
Green colour-coded cap
For EO Sterilization
Features:
3M Clinpro Tooth Creme
Volume: 113g
Strengthens Teeth
Prevents Cavities
Effective Cleaning
Features:
3M Crown Contouring Pliers
Crown Contouring Pliers
Lightweight
Easy to Use
For Enhancing Crown-form
Features:
3M Avagard D Hand Sanitizer with Moisturizers 61% w/v Ethyl Alchohol
Size: 50/500 ml
Kills harmful bacteria
Dries quickly
Gentle on skin
Out of stock
Features:
3M Aplicap Activator/Applier Set
Set Includes 2 Items
For Easy Activation
Lightweight
Durable
Features:
3M Avagard CHG 500 ml Hand Sanitizer – Pack of 10
Size: 500 ml
Rapid skin disinfection
Kills 99.99% of microbes
Protection for longer duration
Out of stock
Features:
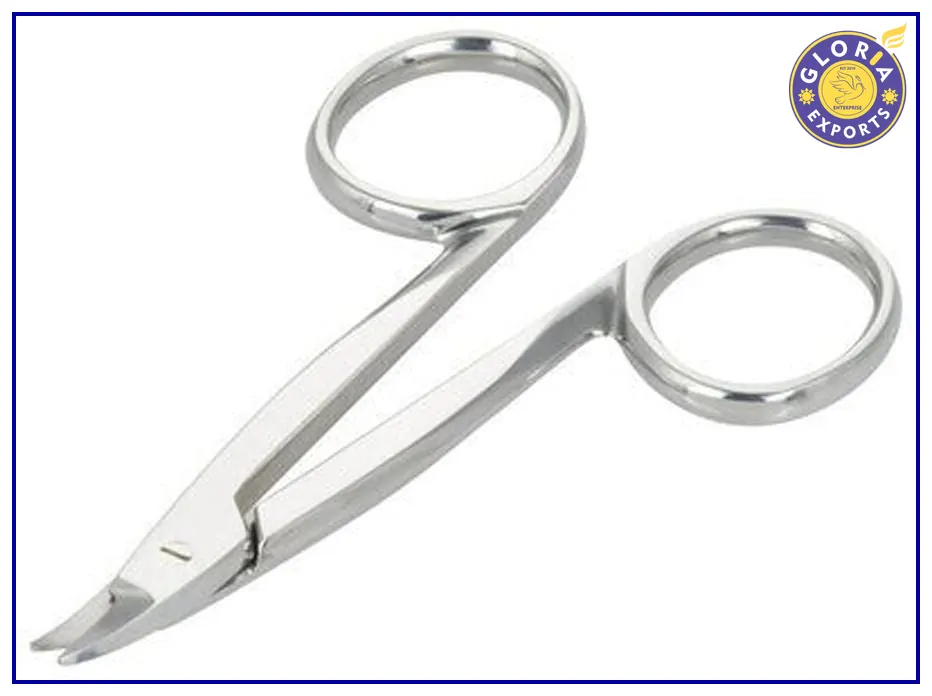
3M Curved Crown Festooning Scissors
Type: Curved
Material: Stainless Steel
Lightweight
Easy to Use
Features:
3M Clinpro Dental Sealant
Long-lasting Protection Against Caries
Colour Change Technology
Low Viscosity
Bonds to Enamel
Features:
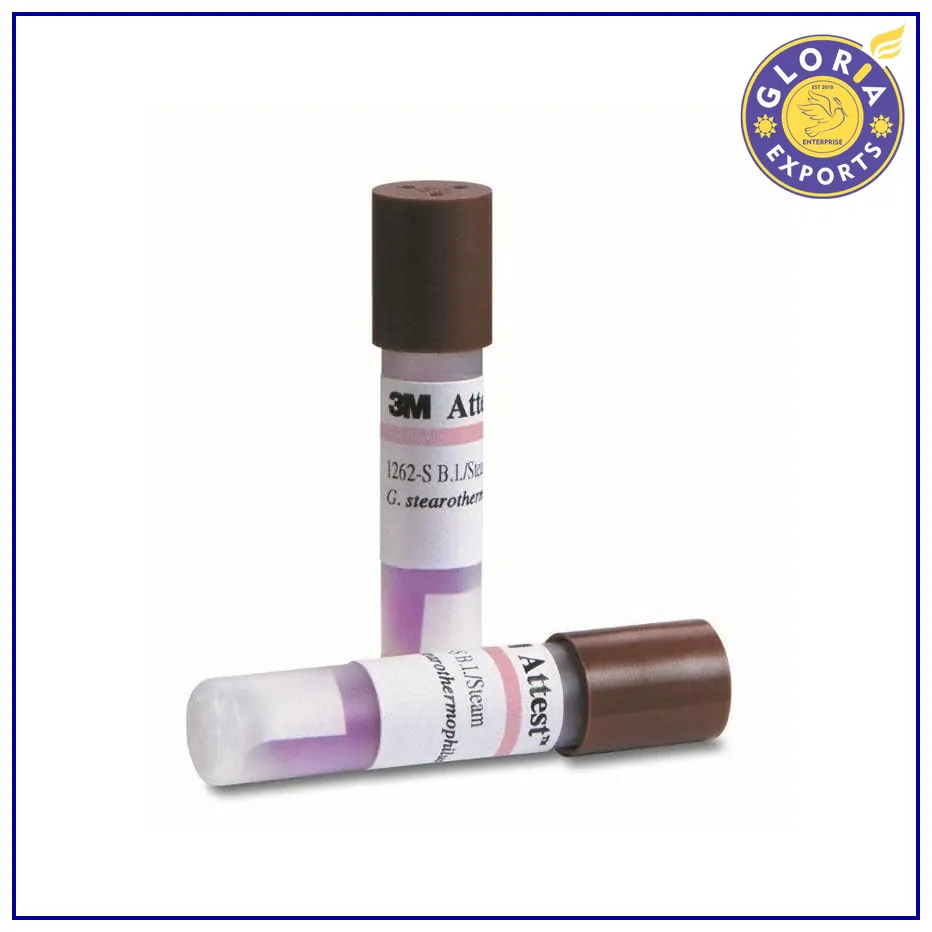
3M Attest Biological Indicator 1262 Test Pack for Steam
Box of 100
Biological indicator
Accurate result
Brown colour-coded cap
Features:
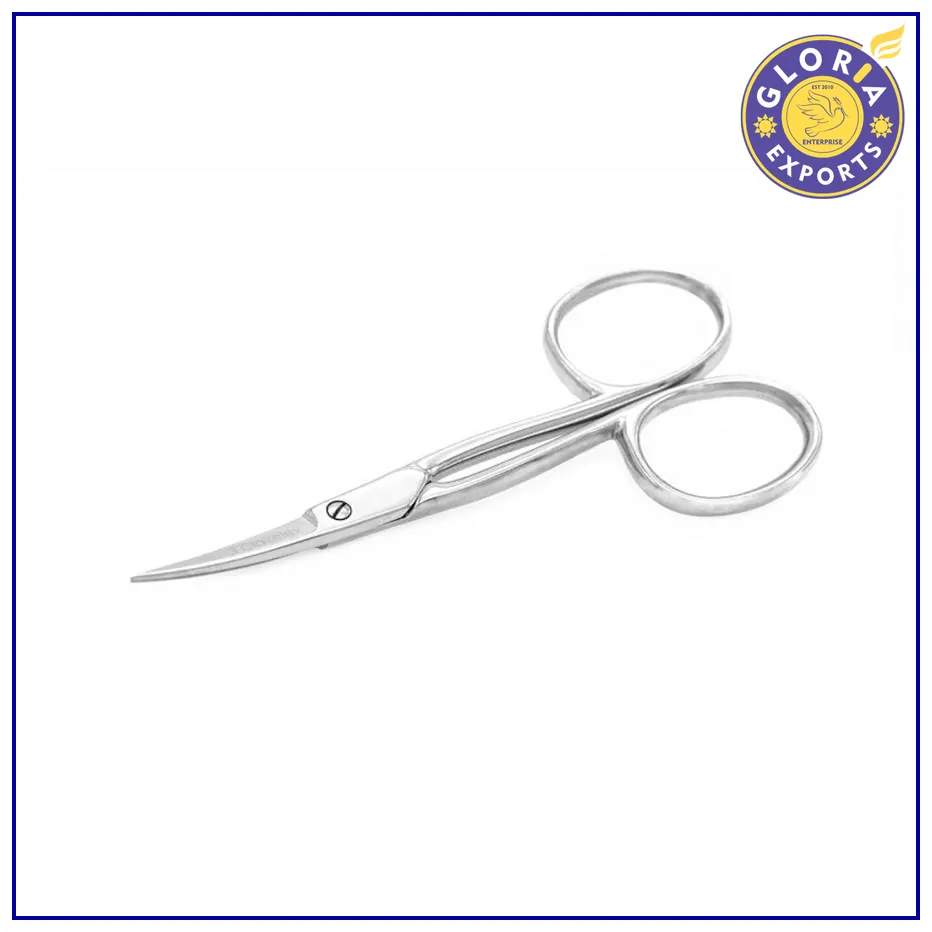
3M Curved Crown Scissors
Type: Curved
Material: Stainless steel
Lightweight
Easy to Use
Features:
3M Cavit G Temporary Filling Material
Volume: 28g
Quick & Void-free Curing
Completely Removable without Burs
Simple to Apply
Features:
3M Adper Easy Bond Dental Adhesive